The Role of Source Data Verification (SDV) and Source Data Review (SDR) in Driving Clinical Trial Data Quality - Medidata Solutions

Source Documentation. Objectives: At the conclusion of this discussion, participants will be able to: –Define source document and source data –Identify. - ppt download
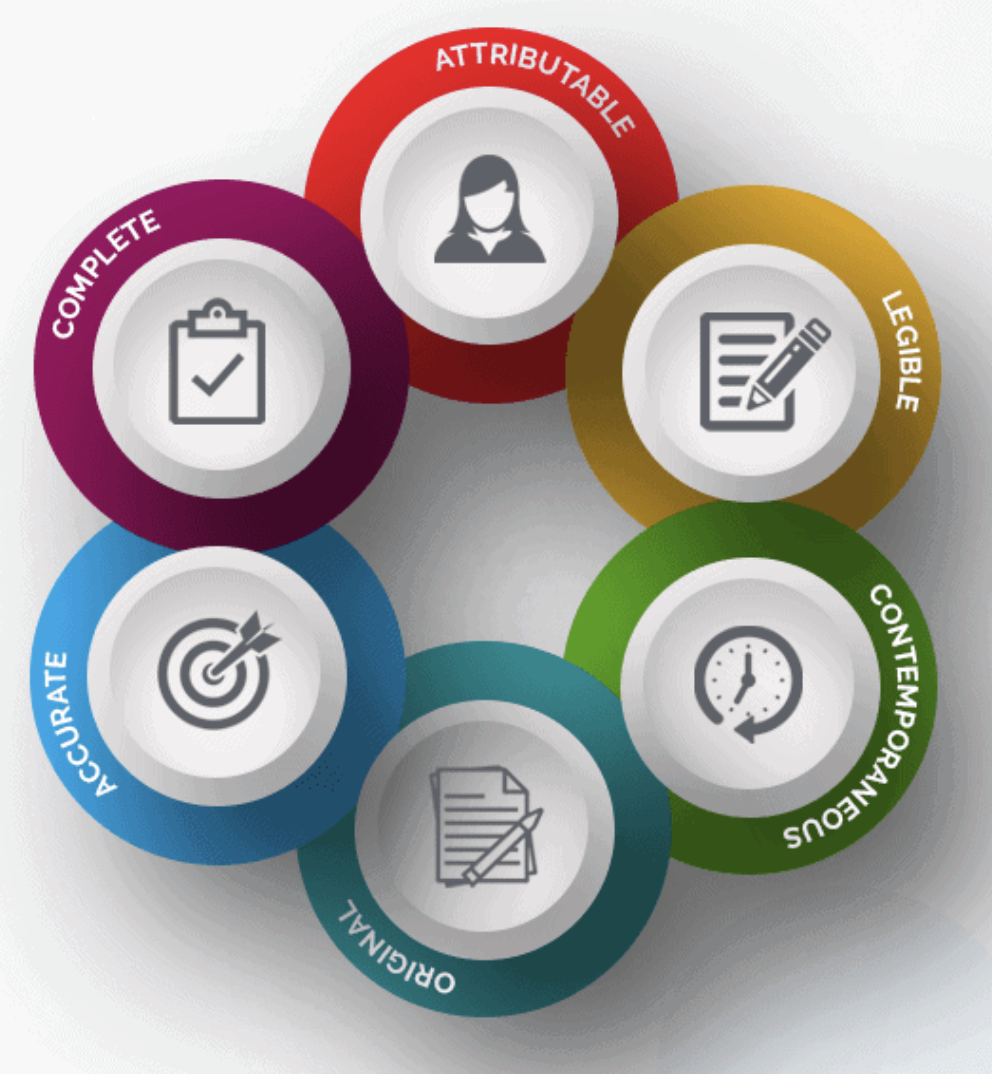
examples of source documents — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification
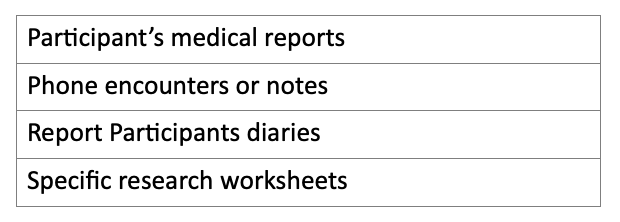
what is a source document — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification

The Necessity of Clinical Research Documentation Training Programs and the Value of Learning from Mistakes - ACRP

CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure | Zenosis – Learning for Life