
REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

Certified Copies and ALCOA-C: Essentials Post ICH GCP E6 (R2) Addendum - Life Science Training Institute
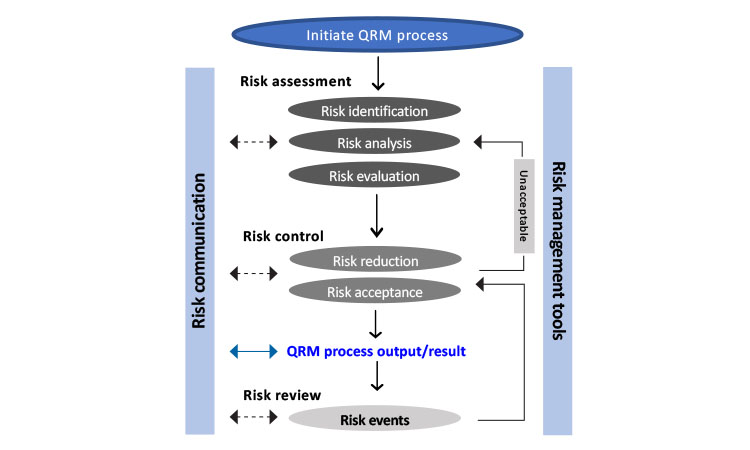
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering

Introduction To Investigators Responsibilities With Good Clinical Practice | PDF | Institutional Review Board | Clinical Trial
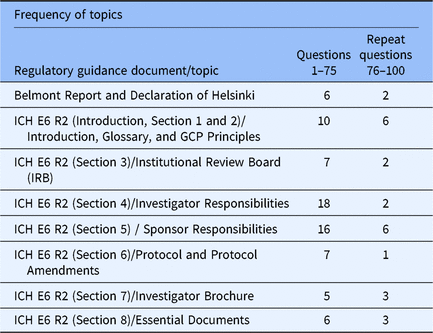
Creating and testing a GCP game in an asynchronous course environment: The game and future plans | Journal of Clinical and Translational Science | Cambridge Core













.jpg)

