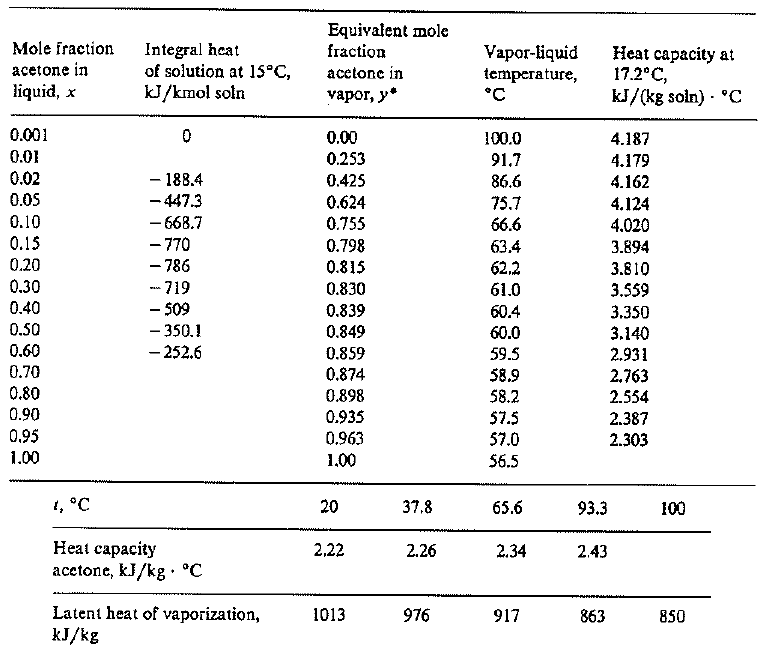
Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram
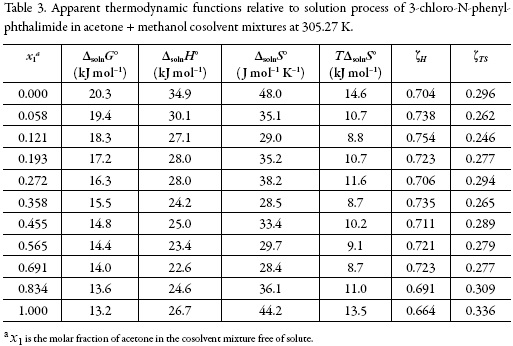
Solution thermodynamics and preferential solvation of 3-chloro-N-phenyl-phthalimide in acetone + methanol mixtures

`Delta_(vap)`S1 of acetone is `93.0 JK^(-1) "mol"^(-1)`.If boiling point of acetone is `56^(@)C`, calculate the heat required for the vaporisation of 1 g of acetone.
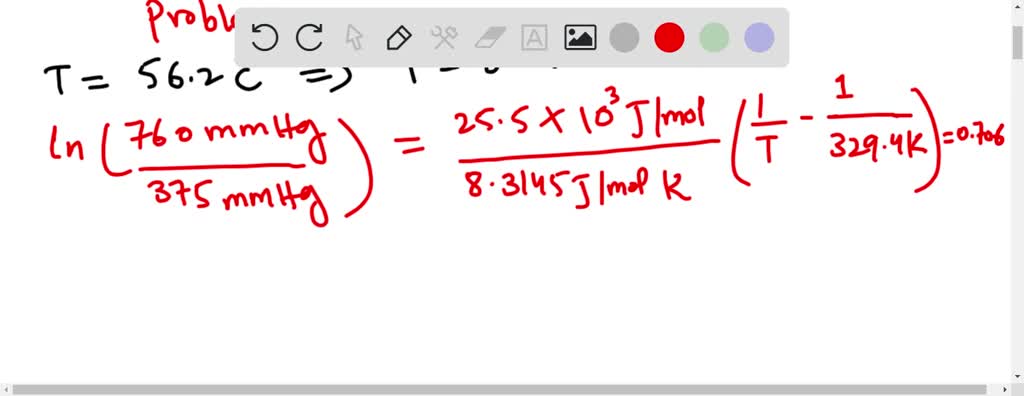
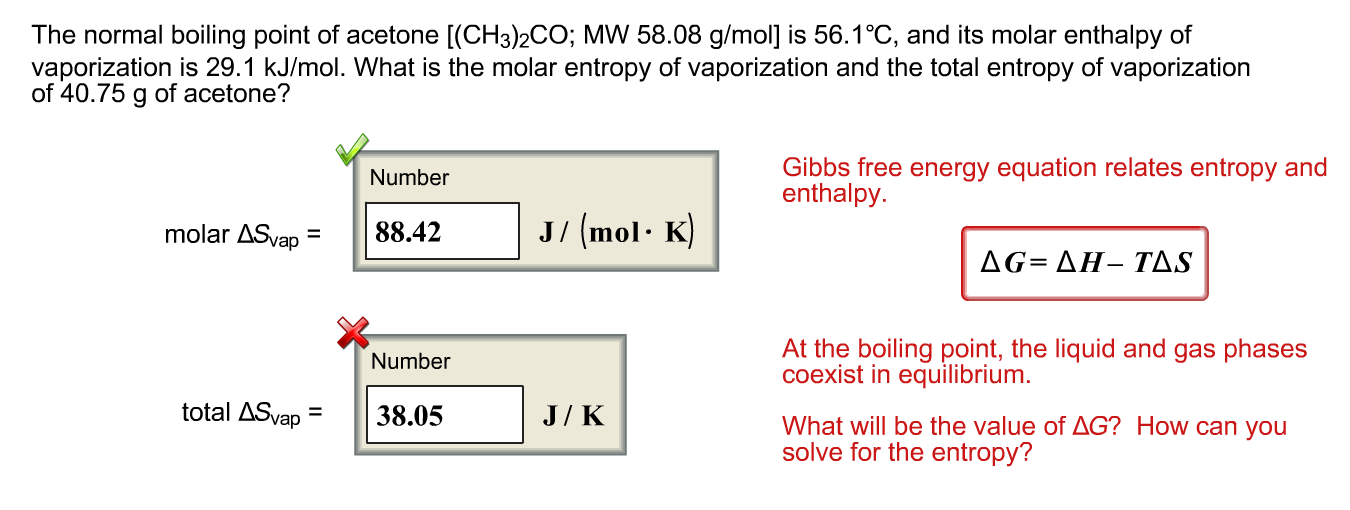
SOLVED:The normal boiling point of acetone is 56.2^{\circ} \mathrm{C}, and the molar heat of vaporization is 32.0 \mathrm{kJ} \mathrm{mol}^{-1}. What is the boiling temperature of acetone under a pressure of 50.0 \mathrm{mmHg} ?

A theoretical analysis on enthalpy of vaporization: Temperature-dependence and singularity at the critical state - ScienceDirect

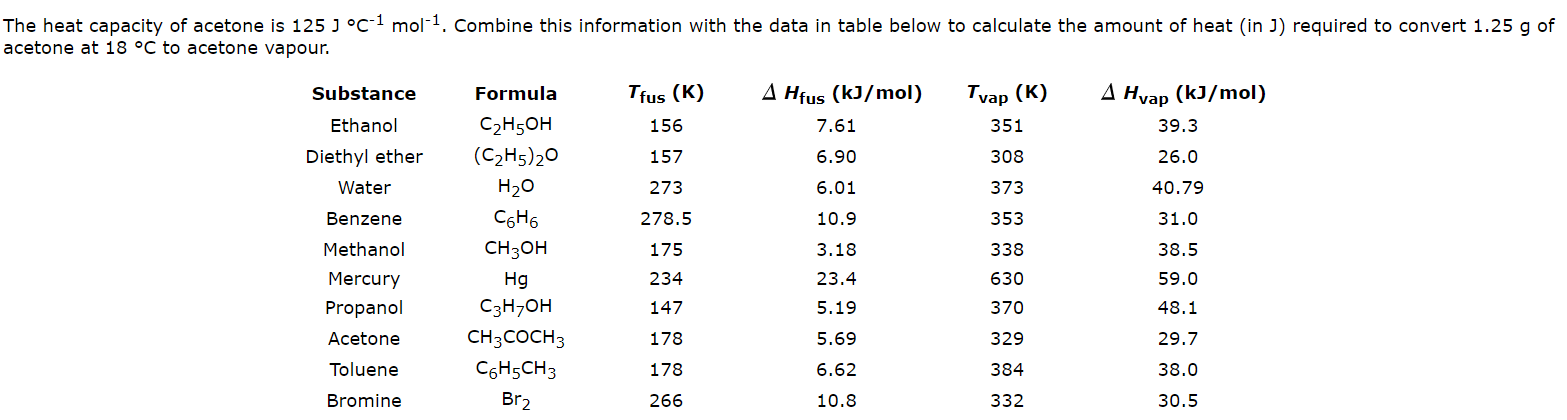
The heat of vaporization of acetone, CH_3COCH_3, at its boiling point is 29.1 kJ/mol. How much energy is required to vaporize 125.0 g of acetone at its boiling point? | Study.com

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram
![PDF] Monte Carlo molecular simulation predictions for the heat of vaporization of acetone and butyramide | Semantic Scholar PDF] Monte Carlo molecular simulation predictions for the heat of vaporization of acetone and butyramide | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f3bfdd077d8b4476ab7043cb9c6c425d0b0d9b0b/3-Table3-1.png)
PDF] Monte Carlo molecular simulation predictions for the heat of vaporization of acetone and butyramide | Semantic Scholar
![PDF] Heat Capacities of Aqueous Solutions of Acetone; 2,5-Hexanedione; Diethyl Ether; 1,2-Dimethoxyethane; Benzyl Alcohol; and Cyclohexanol at Temperatures to 523 K | Semantic Scholar PDF] Heat Capacities of Aqueous Solutions of Acetone; 2,5-Hexanedione; Diethyl Ether; 1,2-Dimethoxyethane; Benzyl Alcohol; and Cyclohexanol at Temperatures to 523 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/87d097be65b5ce058a11c2e59323aff45a229c41/6-Table1-1.png)
PDF] Heat Capacities of Aqueous Solutions of Acetone; 2,5-Hexanedione; Diethyl Ether; 1,2-Dimethoxyethane; Benzyl Alcohol; and Cyclohexanol at Temperatures to 523 K | Semantic Scholar

SOLVED:Equatlon and Table To solve this problem; You will need use the Clausius-Clapeyron equation 4Hyep and this table of boiling points and heat = vaponzation for liquids Liquid Formula Normal boiling Heat

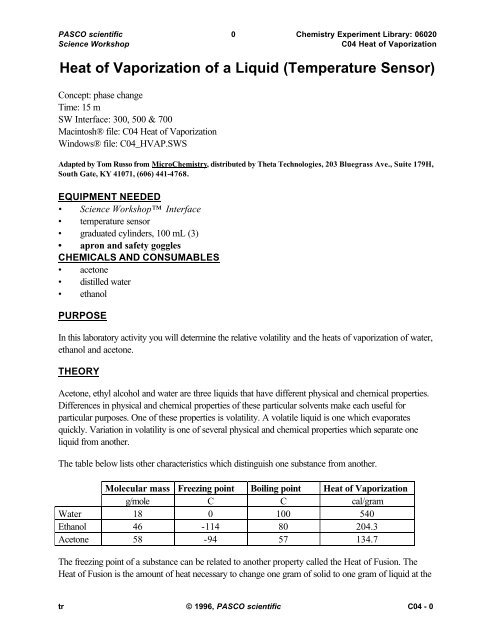
OneClass: 2) The normal boiling point of acetone is 56.5°C At an elevation of 5300 ft, the atmospher...
How to calculate the vapor pressure of acetone at 25.0°C if the enthalpy of vaporization for acetone is 32.0 kJ/mol and the normal boiling point of acetone is 56.5°C - Quora