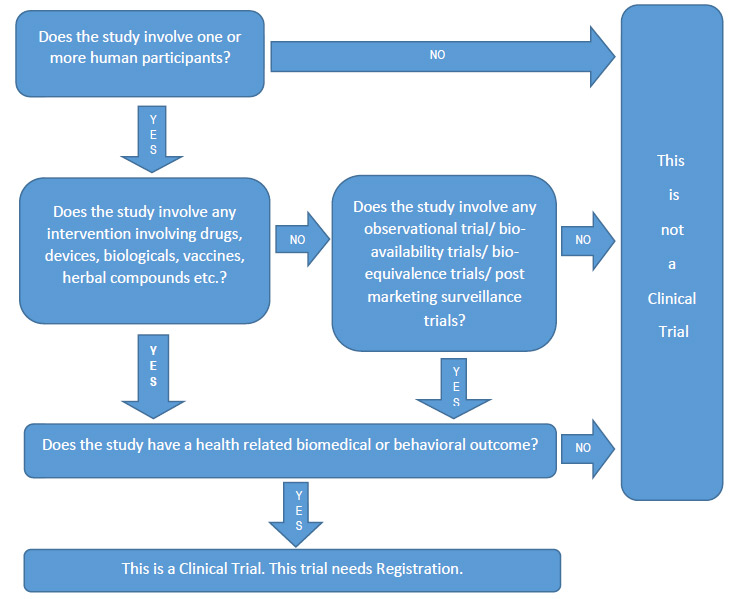
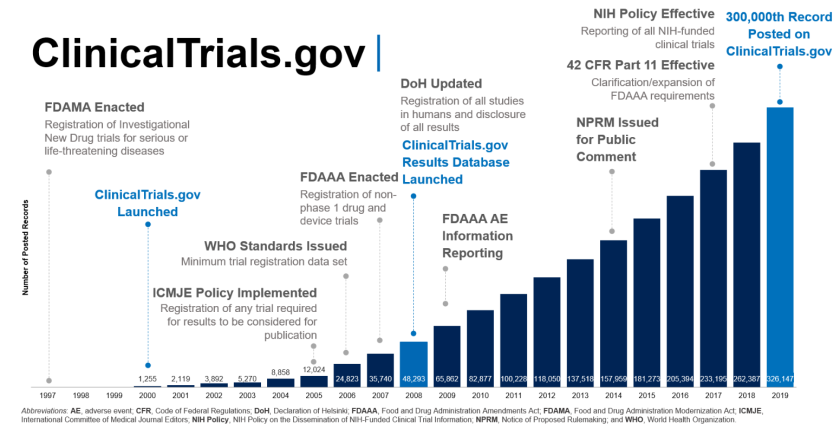
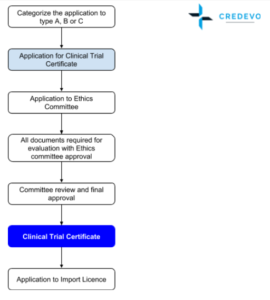
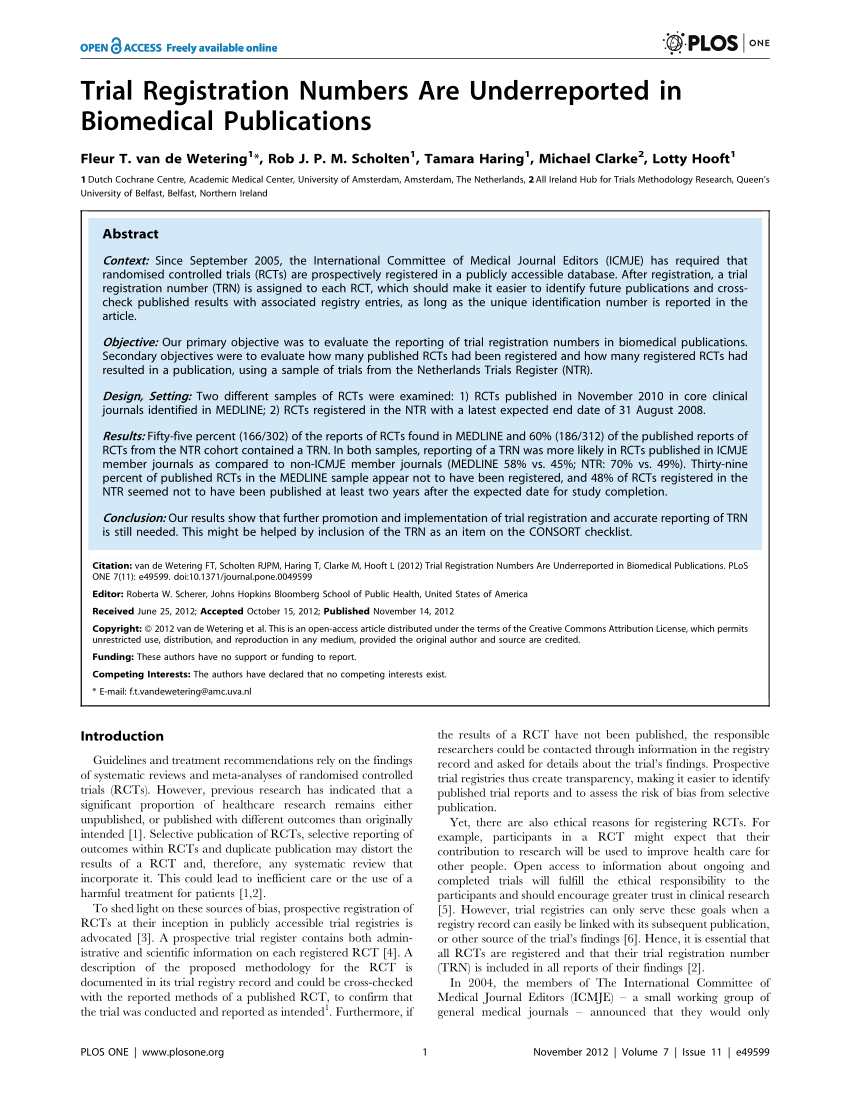
Chinese Clinical Trial Register (ChiCTR) - The world health organization international clinical trials registered organization registered platform
Is Mandatory Prospective Trial Registration Working to Prevent Publication of Unregistered Trials and Selective Outcome Reporting? An Observational Study of Five Psychiatry Journals That Mandate Prospective Clinical Trial Registration | PLOS ONE
PLOS ONE: Is Mandatory Prospective Trial Registration Working to Prevent Publication of Unregistered Trials and Selective Outcome Reporting? An Observational Study of Five Psychiatry Journals That Mandate Prospective Clinical Trial Registration

Figure 1 from Challenges in Administering a Clinical Trials Registry: Lessons from the Clinical Trials Registry-India | Semantic Scholar

Clinical trial registration: a statement from the International Committee of Medical Journal Editors - The Lancet

Retrospective vs. prospective registration of clinical trials – what is the norm? – The Publication Plan for everyone interested in medical writing, the development of medical publications, and publication planning

PDF) CLINICAL TRIAL REGISTRATION: CAN WE ESTABLISH NATIONAL CLINICAL TRIAL REGISTRY OF PAKISTAN? | KMU Journal and Akhtar Sherin - Academia.edu

Figure 3, The process for identifying trial registration for each randomized controlled trial - Selective Outcome Reporting as a Source of Bias in Reviews of Comparative Effectiveness - NCBI Bookshelf