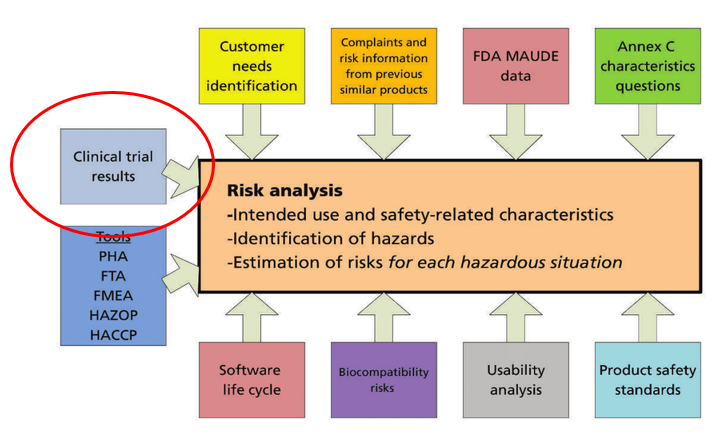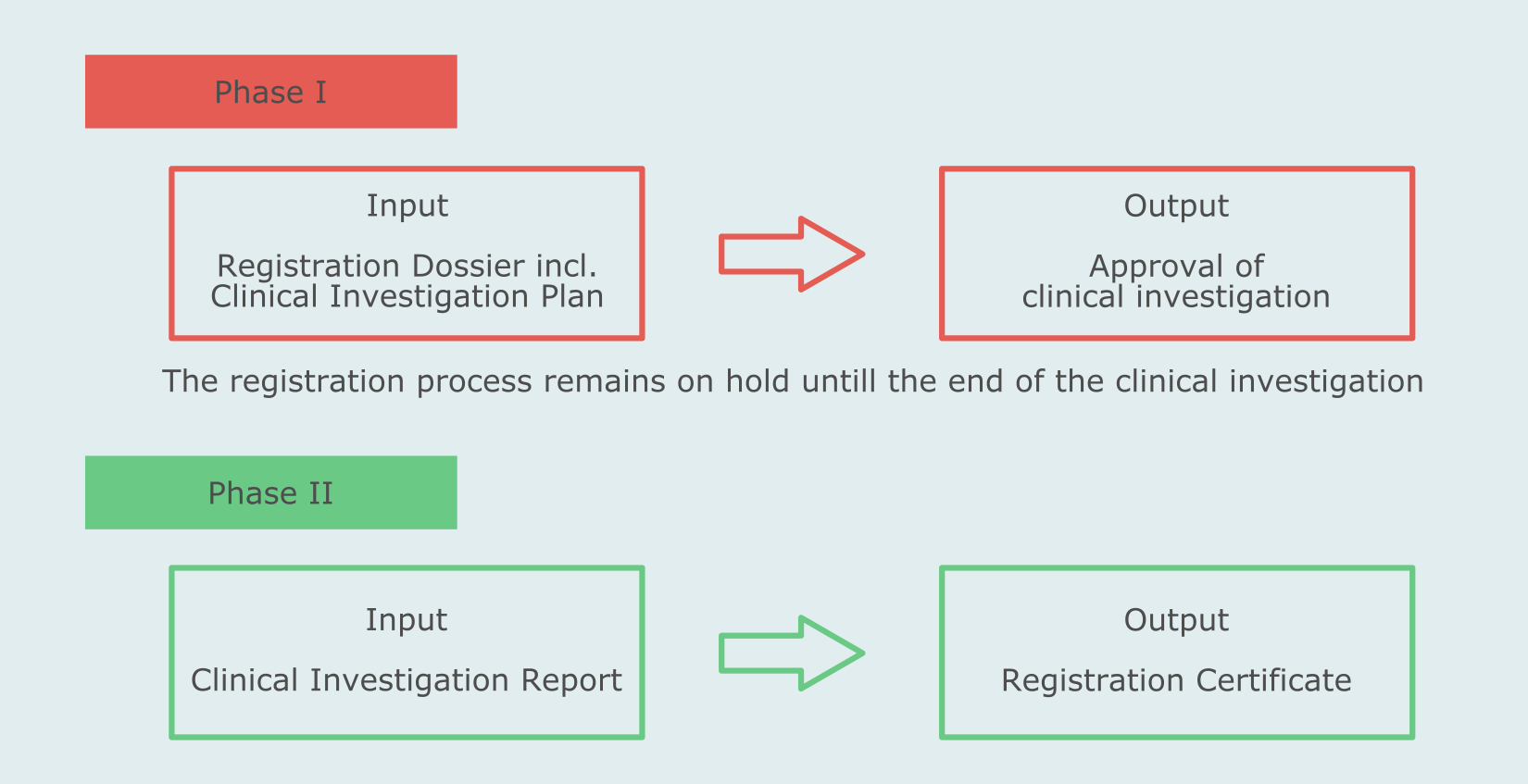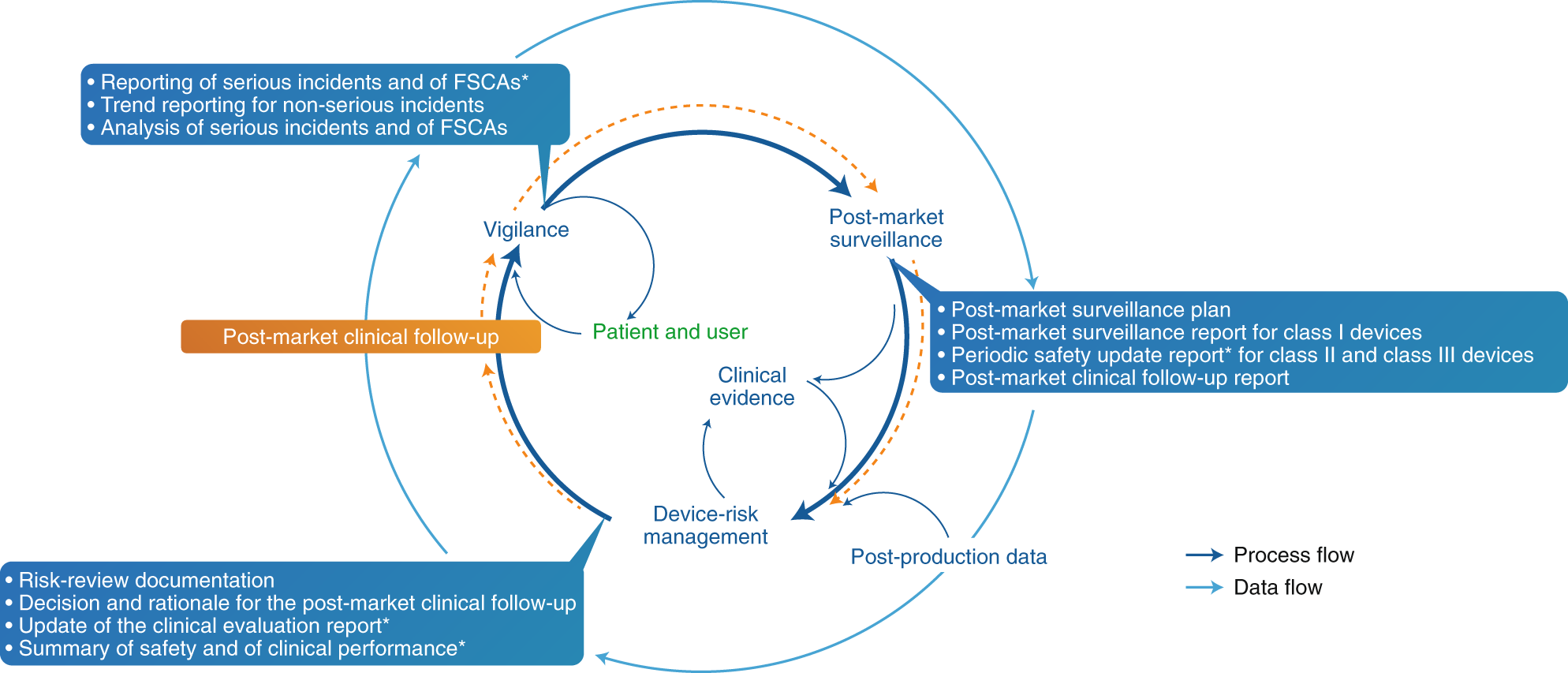
How the new European regulation on medical devices will affect innovation | Nature Biomedical Engineering

Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations - Canada.ca

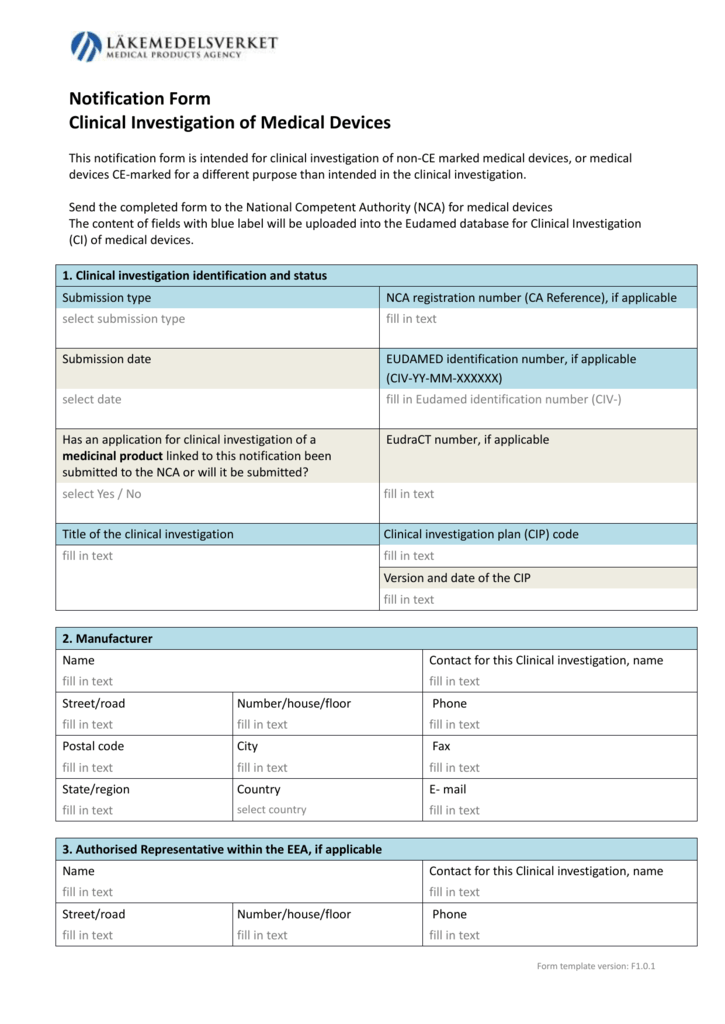
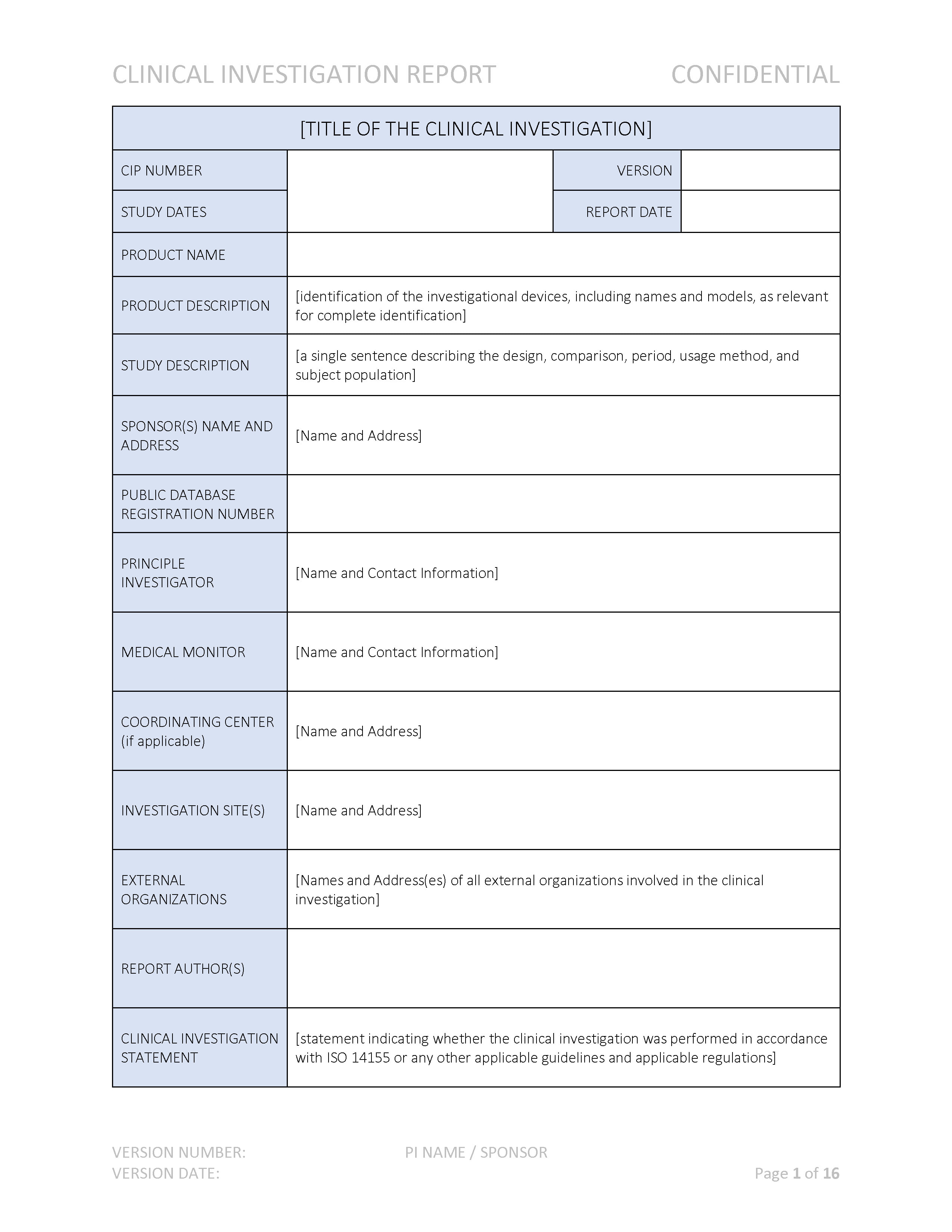
ANSI/AAMI/ISO 14155-2:2003 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans

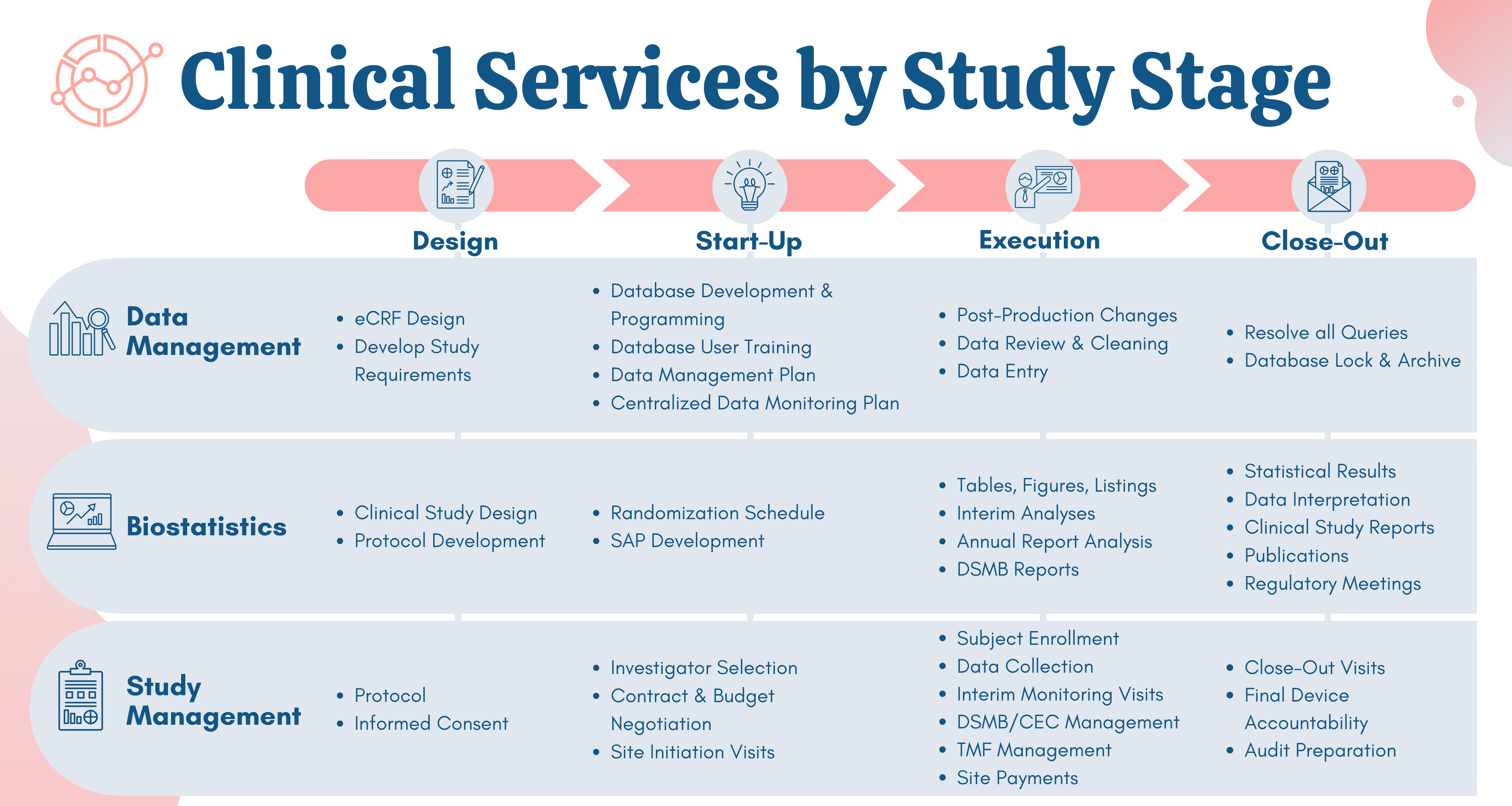
Medical Device Quality, Regulatory and Product Development Blog | Greenlight Guru | QMS Software (4)

Applications for Medical Device Investigational Testing Authorizations Guidance Document - Canada.ca
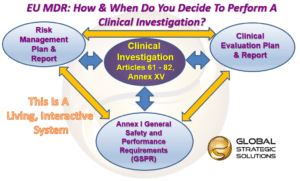
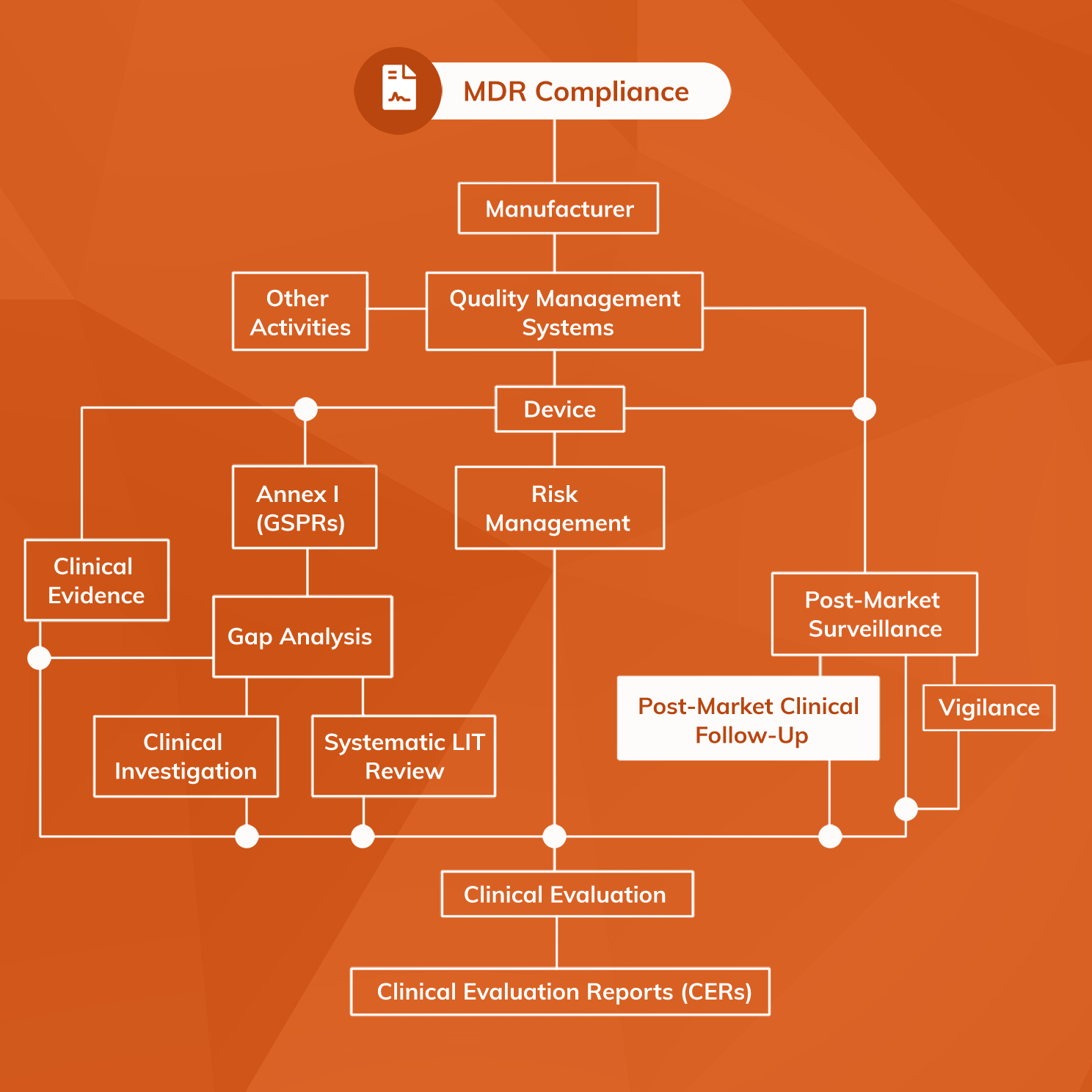
What Is The Difference Between Clinical Evaluation and Clinical Investigation? | Global Strategic Solutions